The atom questions | form five Chemistry
Find The atom examination questions, form five Chemistry in acaproso.com
| # | Question | ||||||
|---|---|---|---|---|---|---|---|
| 1 | (a) Use s,p,d,f notation to designate atomic orbitals with the given pairs of quantum numbers n and l.
(b) For the following sets of quantum numbers , state which are allowable and which are not allowable. Briefly explain your answer. Short answers |
||||||
| 2 | (a) Define the following phrases
(b) Clearly state four postulates of Planck`s quantum theory as derived from black body radiation. Short answers |
||||||
| 3 | A nucleus of a certain element is presented as Mathematical Calculation |
||||||
| 4 | Sketch a hydrogen spectral series based on Bohr atom energy level. Short answers |
||||||
| 5 |
Calculate the relative atomic mass of an element. Mathematical Calculation |
||||||
| 6 |
Mathematical Calculation |
||||||
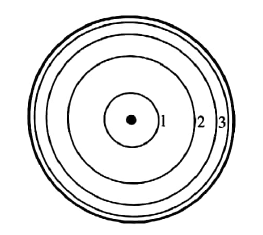
| 7 | Study carefully the Bohr`s atomic model of hydrogen atom shown below then answer the questions that follow.
Long answers |
||||||
| 8 | The energy difference between ground and excited state atoms of a certain element was found to be Mathematical Calculation |
||||||
| 9 | Dalton`s atomic theory consists of four main postulates. For each of the two postulates given below, briefly describe an experiment or discovery which is against the postulate.
Long answers |
||||||
| 10 | State four postulates of Dalton’s atomic theory. Short answers |