Two component liquid systems questions | form five Chemistry
Find Two component liquid systems examination questions, form five Chemistry in acaproso.com
| # | Question |
|---|---|
| 1 |
Short answers |
| 2 | Briefly explain the meaning of the following phrases.
Long answers |
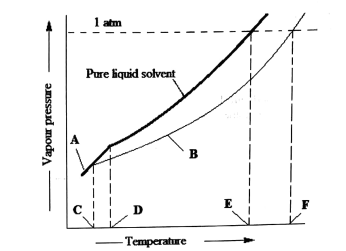
| 3 | Study the following liquid-vapour phase diagram and then answer the questions that follow.
Short answers |
| 4 | When water and ice are mixed , the temperature of the mixture is 0oC, but , if methanol (CH3OH) and ice are mixed , a temperature of +10oC is readily attained. Explain why the two mixtures show such different temperature behaviours. Short answers |
| 5 | Study the following diagram and then answer the subsequent questions.
Short answers |
| 6 | Nitrobenzene (C6H5NO2) and water form a mixture of immiscible liquids which boils at 99oC. Calculate the percentage by mass of nitrobenzene in the distillate when the mixture is distilled at 1.013x105Pa given that the vapour pressure of water at 99oC is 9.749x104Pa. Show your work clearly including manipulations of units. [Molar masses: nitrobenzene = 123g mol-1, water =18g mol-1]. Mathematical Calculation |
| 7 | Water (b.p 100oC) and phenylamine (b.p 184oC) form a mixture of immiscible liquids that boils at 98oC. With the aid of a diagram , show how steam distillation of this mixture can be achieved. Mathematical Calculation |
| 8 | Show how the equation for the partition law of solute `x` dissolved in two immiscible solvents A and B will be represented when solute `x`:
Mathematical Calculation |
| 9 | Fifty (50) grams of the acid are dissolved in one litre (1000cm3) of water. The distribution coefficient of the acid between ether and water is 3. A volume of 1000cm3 of ether is available for use in the extraction process. Two experiments were performed to extract acid from water. In the forst experiment, 1000cm3 of ether were used once, i.e., single extraction . In the second experiment, two extractions were performed , each using 500cm3 of ether. Compare the amounts of the acid left in the aqueous solution in each case and recommend the best method to extract the acid from water. Mathematical Calculation |