Chemical kinematics, equilibrium and energetics questions | form three Chemistry
Find Chemical kinematics, equilibrium and energetics examination questions, form three Chemistry in acaproso.com
| # | Question |
|---|---|
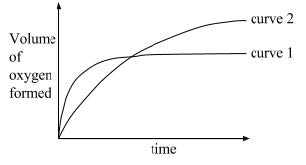
| 1 | In the graph below, curve 1 was obtained from the decomposition of 100 cm3 of 1.0M hydrogen peroxide solution catalysed by manganese (IV) oxide,
Which alteration/change to the original experimental conditions would produce curve 2? Multiple choices |
| 2 | Two experiments were carried out using the same mass of magnesium ribbon and the same volume of acids of the same concentration. The acids were 1M hydrochloric acid and 1M ethanoic acid. The results were as shown in the following figure: (a) If the experiments were conducted within the same time, is there a difference in volumes of hydrogen gas collected at the same room temperature and pressure? Give reasons for your answer. Short answers |
| 3 | A student attempted to prepare hydrogen gas by reacting zinc metal with dilute sulphuric acid. In this experiment zinc metal granules of about 0.5 cm diameter and 0.20 moles of acid were used. The rate of formation of hydrogen gas was found to be slow. Short answers |
| 4 | State and describe the type of reaction in the following chemical equations: Short answers |
| 5 | In the following equilibrium equation,
Multiple choices |
| 6 | The following equation shows the reaction between hydrogen and iodine gas to form hydrogen iodide gas, Short answers |